"Executive Summary Healthcare Regulatory Affairs Outsourcing Market Market Size, Share, and Competitive Landscape
The global Healthcare Regulatory Affairs Outsourcing market size was valued at USD 6.42 billion in 2024 and is projected to reach USD 14.49 billion by 2032, with a CAGR of 10.71% during the forecast period of 2025 to 2032. To attain knowhow of market landscape, brand awareness, latest trends, possible future issues, industry trends and customer behavior, the finest Healthcare Regulatory Affairs Outsourcing Market Market research report is very crucial. The report also identifies and analyses the intensifying trends along with major drivers, challenges and opportunities in the market. This market report is a source of information about Healthcare Regulatory Affairs Outsourcing Market Market industry which puts forth current and upcoming technical and financial details of the industry to 2029. Global Healthcare Regulatory Affairs Outsourcing Market Market business report has been formed with the appropriate expertises that utilize established and unswerving tools and techniques such as SWOT analysis and Porter's Five Forces analysis to conduct the research study.
The high quality Healthcare Regulatory Affairs Outsourcing Market Market business report encompasses a range of inhibitors as well as driving forces of the market which are analysed in both qualitative and quantitative manner so that readers and users get precise information and insights. All the data and statistics covered in this report are backed up by latest and proven tools and techniques such as SWOT analysis and Porter's Five Forces Analysis. For in depth perceptive of market and competitive landscape, the report serves a lot of parameters and detailed data. The universal Healthcare Regulatory Affairs Outsourcing Market Market report is prepared by performing high level market research analysis of key marketplace segments to identify opportunities, challenges, drivers, and market structures for the clients.
See what’s driving the Healthcare Regulatory Affairs Outsourcing Market Market forward. Get the full research report:
https://www.databridgemarketresearch.com/reports/global-healthcare-regulatory-affairs-outsourcing-market
Healthcare Regulatory Affairs Outsourcing Market Industry Landscape
Segments
- Based on service, the healthcare regulatory affairs outsourcing market can be segmented into regulatory writing and publishing, clinical trial applications, product registrations, regulatory submissions, and other services.
- By end-user, the market can be categorized into pharmaceutical, biotechnology, medical devices, and other end-users.
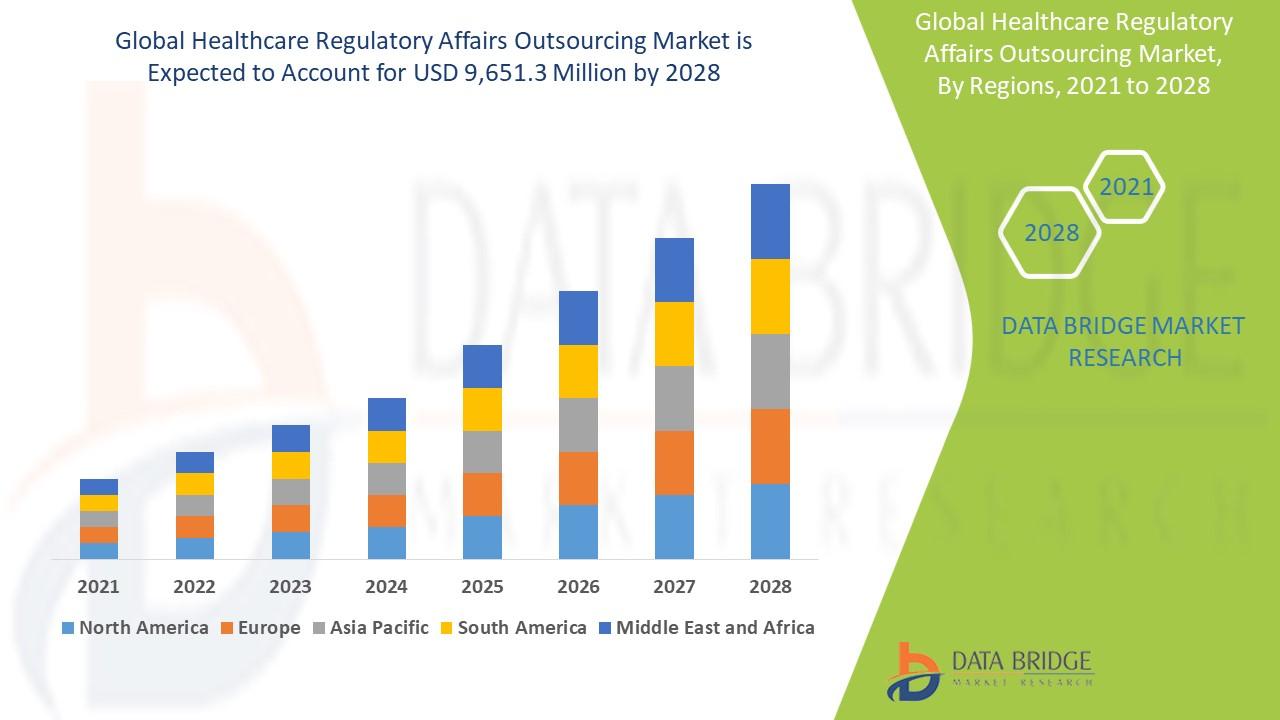
- On the basis of geography, the market can be divided into North America, Europe, Asia-Pacific, South America, and Middle East & Africa.
The increasing complexity of regulatory requirements, along with the growing emphasis on compliance and quality standards, is driving the demand for healthcare regulatory affairs outsourcing services. Companies in the pharmaceutical, biotechnology, and medical devices industries are increasingly outsourcing regulatory affairs activities to specialized service providers to ensure timely approvals and compliance with regulatory guidelines. The regulatory writing and publishing segment is expected to witness significant growth due to the rising number of new drug approvals and the need for accurate and compliant documentation.
Market Players
- Some of the key players in the global healthcare regulatory affairs outsourcing market include Parexel International Corporation, ICON plc, Accell Clinical Research, Clinilabs Inc., Wuxi AppTec, Pharmaceutical Product Development, LLC, and Promedica International.
- Other notable market players are Freyr Solutions, Covance Inc., Criterium, and Novotech.
Market players in the healthcare regulatory affairs outsourcing sector are focusing on expanding their service offerings, enhancing their regulatory expertise, and entering into strategic partnerships to strengthen their market presence. The competitive landscape is characterized by collaborations between service providers and pharmaceutical companies to streamline regulatory processes and expedite product approvals. With the increasing globalization of clinical trials and the emergence of new markets, regulatory affairs outsourcing is expected to play a pivotal role in ensuring compliance with diverse regulatory requirements.
For more detailed insights, refer to The healthcare regulatory affairs outsourcing market is witnessing steady growth driven by the increasing complexity of regulatory requirements and the focus on compliance and quality standards within the pharmaceutical, biotechnology, and medical devices industries. As companies strive for timely approvals and adherence to regulatory guidelines, they are turning to specialized service providers for regulatory affairs activities. The emphasis on accurate and compliant documentation is boosting the demand for services such as regulatory writing and publishing, clinical trial applications, product registrations, and regulatory submissions.
Key players in the global healthcare regulatory affairs outsourcing market, including Parexel International Corporation, ICON plc, and Wuxi AppTec, are continuously enhancing their service offerings and regulatory expertise. Strategic partnerships and collaborations between service providers and pharmaceutical companies are becoming prominent in the market landscape, aimed at streamlining regulatory processes and accelerating product approvals. These partnerships allow for greater efficiency in navigating the intricacies of diverse regulatory requirements, especially with the increasing globalization of clinical trials and the exploration of new markets.
In addition to the established market players, emerging companies like Freyr Solutions, Criterium, and Novotech are also making inroads in the healthcare regulatory affairs outsourcing sector. These players are leveraging technological advancements and industry expertise to offer innovative solutions tailored to the evolving regulatory landscape. As regulatory authorities across different regions continue to update and modify guidelines, the need for agile and compliant regulatory affairs outsourcing services will remain crucial for companies seeking market approvals and commercial success.
The market dynamics of the healthcare regulatory affairs outsourcing sector are shaped by ongoing regulatory developments, technological advancements, and the evolving needs of the pharmaceutical, biotechnology, and medical devices industries. As the demand for outsourcing services continues to grow, market players will need to adapt to new challenges and opportunities in order to stay competitive and meet the changing requirements of their clients. Strategic differentiation, regulatory acumen, and efficient collaboration will be key factors determining the success of players in this dynamic and critical market segment.The global healthcare regulatory affairs outsourcing market is poised for significant growth due to the increasing complexity of regulatory requirements and the focus on compliance and quality standards within the pharmaceutical, biotechnology, and medical devices industries. Companies in these sectors are turning to specialized service providers to navigate the intricate landscape of regulatory affairs, ensuring timely approvals and adherence to guidelines. This trend is driving the demand for services such as regulatory writing and publishing, clinical trial applications, product registrations, and regulatory submissions.
Key market players like Parexel International Corporation, ICON plc, and Wuxi AppTec are continuously enhancing their service offerings and regulatory expertise to meet the evolving needs of clients. The strategic partnerships and collaborations between service providers and pharmaceutical companies are becoming more prevalent, aimed at streamlining processes and expediting product approvals. These alliances enable companies to navigate the complexities of diverse regulatory requirements efficiently, especially in the current climate of globalization and exploration of new markets.
Emerging players in the market, such as Freyr Solutions, Criterium, and Novotech, are leveraging technological advancements and industry expertise to offer innovative solutions tailored to the changing regulatory landscape. These companies are gaining traction by providing agile and compliant outsourcing services that align with the dynamic needs of their clients. As regulatory authorities worldwide update guidelines, the demand for regulatory affairs outsourcing services will continue to be crucial for companies seeking market approvals and successful commercialization strategies.
The market dynamics of healthcare regulatory affairs outsourcing are shaped by a combination of regulatory developments, technological advancements, and industry requirements. Market players must adapt to these changing dynamics by focusing on strategic differentiation, regulatory acumen, and efficient collaboration to remain competitive in the sector. With an increasing emphasis on compliance and quality standards, service providers will need to stay abreast of regulatory changes and adopt innovative approaches to meet the diverse needs of their clients effectively. Overall, the healthcare regulatory affairs outsourcing market presents opportunities for growth and innovation, with strategic partnerships and regulatory expertise playing a pivotal role in driving success in this critical segment.
Review the company’s share in the market landscape
https://www.databridgemarketresearch.com/reports/global-healthcare-regulatory-affairs-outsourcing-market/companies
Healthcare Regulatory Affairs Outsourcing Market Market – Analyst-Ready Question Batches
- What regulatory frameworks govern this Healthcare Regulatory Affairs Outsourcing Market Market industry?
- What proportion of sales come from promotions or discounts?
- What is the average shelf life of the Healthcare Regulatory Affairs Outsourcing Market Market product?
- How important is personalization in this Healthcare Regulatory Affairs Outsourcing Market Market?
- What are the trends in user-generated content for Healthcare Regulatory Affairs Outsourcing Market Market?
- What is the average profit margin per unit?
- What’s the demand trend across income groups?
- What portion of sales comes from Tier II & III cities?
- Which retailers dominate product placement?
- What’s the average customer acquisition cost for Healthcare Regulatory Affairs Outsourcing Market Market?
- What new market segments are emerging?
- What are the effects of digital transformation?
- Which trends are influenced by Gen Z consumers?
- What are the implications of the circular economy for Healthcare Regulatory Affairs Outsourcing Market Market?
Browse More Reports:
Global Dimethyl Ether Market
Global Hair Wigs and Extension Market
Global Underwater Robotics Market
Global Colored Gemstones Market
Global Aspirin Market
Global Customer Data Platform Market
Global Quantum Dot Solar Cell Market
Sri Lanka Elderly Care Market
Global Camel Dairy Market
Global Freight Forwarding Market
Global Parkinson’s Disease Treatment Market
Global Sports Technology Market
Global Cannabis Market
Global Flavored Water Market
Europe Medicinal Mushroom Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- [email protected]
"